What Are pH Strips? Their Indicators and How They Work
May 12, 2022
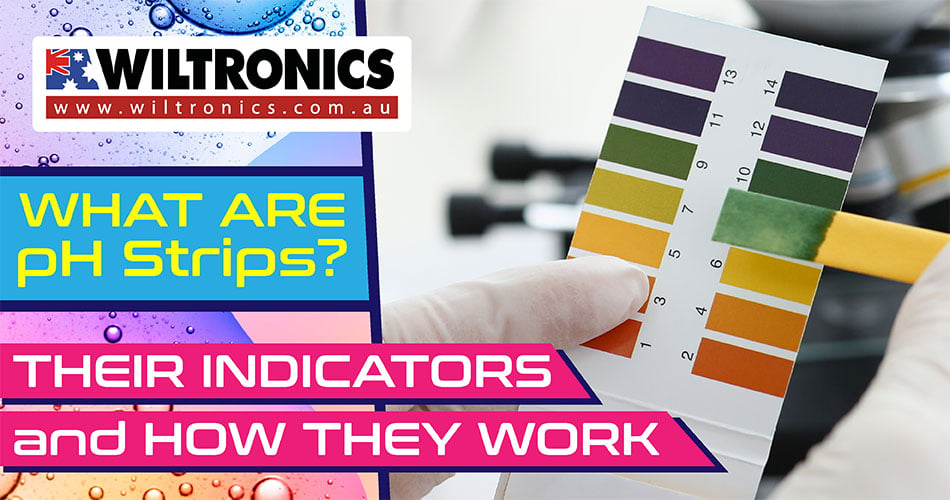
Apart from colour-changing pieces of paper, what are pH strips?
A balanced pH relies on the accuracy of the pH measurement. The value of this plays a significant role, controlling the availability of:
- Nutrients in agricultural production
- Biological functions
- Microbial activity
- Chemical behaviour
- Environmental monitoring
The pH balance is everywhere—it is an important indicator for our body, animal species, the resources we use, and more. Hence why it is vital to do a pH test, be it on water and soil, or food and beverage products.
pH measurement tools have been, and continue to be, widely used as an accurate measure of hydrogen ion concentration. One of the common methods for this monitoring job is pH strips.
In a previous article, we unpacked the essentials about pH meters. This time, we are going to highlight the paper version, answering questions like: what are pH strips, the indicators, and how do they work?
The Basics of pH
The abbreviation pH stands for potential hydrogen. It tells us how active the hydrogen ion is, measuring acidity or alkalinity in a solution.
This degree of hydrogen ion activity is expressed as pH level—a term that is widely used in chemistry, biology, and agronomy. It uses a logarithmic scale of 0 to 14.
A pH of 0 represents the most acidic level, meaning that the substance at hand has more hydrogen ions in it. The opposite end of the scale, a pH of 14, represents the most basic or alkaline level. This means that the substance has more oxygen ions.
And to put things into perspective, a pH of 7 is neutral. This means that your solution is as pure as it can get; it is the ideal pH for water in our bodies.
Here is the general breakdown of pH levels:
- Neutral solution: pH = 7
- Acidic solution: pH < 7
- Basic solution: pH > 7
A well-balanced pH level is a necessity, not only for water but also for health and practical reasons.
Identifying pH Levels in Solutions
Let us use water as a great example. Water with a low pH can negatively affect plumbing, causing pipes to deteriorate.
This results in water tasting and looking abnormal, let alone unhealthy.
Another one: battery acid is an example of a pH of 0. Whereas, a drain cleaner would be a pH of 14.
It is also important to know the pH when you want to determine whether a disinfectant is still effective. Luckily, it is easy to test the pH levels when you have the right measurement tool, such as pH strips.
What are pH Strips?
pH strips are made of litmus paper with which you can measure the hydrogen ion concentration of a liquid. The substance causes the strips to show a different colour at different acidities.
The standard scale is from 0 to 14, where 0 is very acidic and 14 is very alkaline. But some pH test strips can measure only acid or only alkaline substances.
There are a variety of litmus paper-type pH strips available. They differ by sensitivity and what range of pH they are designed for.
The more sensitive the pH strip, the smaller the range in which it works. Depending on what range you want and how sensitive you need the strip to be, you adjust your indicators.
You can have a pH strip with a range of 1 to 14, but not very sensitive. Or opt for one with a range of 5.5 to 8.8 that can measure changes as small as 0.25 in pH.
Now that you know what are pH strips and how they work, it is time to know how to use them!
How To Use pH Strips?
Dip the pH strip in the fluid which you want to control for two seconds, then wait for the result for ten seconds. When the strip contacts an acidic or alkaline substance, it discolours.
The more acidic the solution, the redder the strip becomes. In contrast, the more alkaline it is, the bluer the strip becomes.
Imagine you want to test your water pH level, for instance. If your water tastes metallic or has a red or blue tint to it, you could have acidic water.
Depending on the pH level, it will turn into a certain colour. Use the indicator scale that comes with the strips to see the colour chart. This can help you determine how acidic or alkaline the solution you measured is.
General Colour Changes
When a pH strip is dipped into a vial of an acidic solution, such as hydrochloric acid, it turns red. But when it is dipped into an alkaline like sodium hydroxide, it turns into a blue or greenish colour.
Meanwhile, when the strip is in the presence of pure water or neutral, it turns purple.
Note: If you are using litmus paper, it only turns two colours: red for acids and blue for bases.
pH Indicators Chart
pH indicators are weak acids or weak alkaline (bases) that change colour at specific pHs. They come in the form of liquid dyes and dye-infused paper strips.
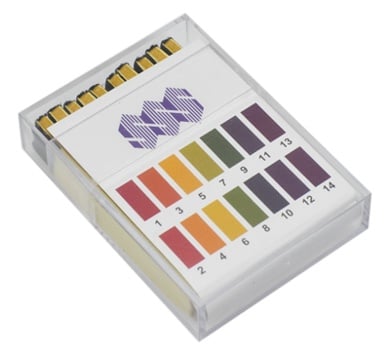
Have a look at these Universal pH Indicator Strips (ME4300). They are full range (0-14) pH indicator strips supplied in 10 books of 20.

For a soil pH test kit, you also might want to check out our Soil Test Kit (ME4364). It makes an excellent addition to your gardening essentials.
pH indicators are also available in charts, which display the colours of ten common indicators over the entire pH range. Having a chart like this can help you identify colour changes easily and know whether the solution is acidic, neutral, or alkaline.
What are pH Strips: The Bottom Line
pH strips are more affordable and are very useful to quickly determine whether a solution is acidic, neutral or alkaline. Understanding what pH strips are and how they work can help us learn the importance of pH.
We hope this quick guide has answered your questions regarding what are pH strips!
© Electrotech Brands Pty Ltd 2022
Write a Comment
You must be logged in to post a comment.


Thanks, really helped me with my Grade 8 science project about “What is the pH level of household liquids, and how do the pH strips work?”!